Outsourcing The Cure: How Our Dependence On Foreign Manufactured Pharmaceuticals Is Making Us Sick
A crippled domestic supply chain combined with an overreliance on foreign pharmaceuticals has positioned China as the world’s pharmacy and precariously perched us on the precipice of catastrophe
“I got an automated call from CVS saying we don’t have your medication, and I was on hold forever. The next three hours were beyond hellacious,” said Jennifer Cronin, an Ashland mother desperately trying to fill an amoxicillin prescription for her 4-year old son’s ear infection. Cronin then called her pediatrician, who sent the pharmacy a prescription for an alternate antibiotic. “We drove all the way to the CVS, waited in line, got up to the counter, and they said,’ We don’t have that medication either,’” she said.
For the next several hours, Cronin and her husband frantically worked the phones, calling pharmacies and their pediatrician. Finally they were able to fill the prescription for amoxicillin at another store.
Although this story was featured prominently in the news media, such occurrences are becoming startlingly commonplace these days.
Why This Matters
According to a 2023 US Government report, our pharmaceutical manufacturing sovereignty is on life support:
Drug shortages are increasing, lasting longer, and impacting patient care
The COVID-19 pandemic exacerbated already lean supply lines and left providers scrambling for alternative drug options
A staggering 87 percent of generic Active Pharmaceutical Ingredient (API) manufacturing is located overseas
Between 2021 and 2022, new drug shortages increased by a whopping 30 percent.
At the end of 2022, the United States experienced a record five-year high of 295 active drug shortages
During a time of international upheaval and rising tensions with China, overreliance on foreign sources for critical drugs, their starting materials and limited domestic manufacturing capabilities is a recipe for disaster and poses a grave health and national security risk
In 2016, an investigation by China’s own Food and Drug Administration (SFDA) found that 80 percent of clinical trial data submitted by Chinese companies to regulators to gain approval for new drugs was fabricated.
Shortages of critical medications continue to rise—including drugs used in hospital emergency rooms, cancer drugs, prescription medications, and even common over-the-counter treatments like children’s cold and flu medicine.
Between 2010 and 2015, the number of Chinese based pharmaceutical ingredient manufacturers registered with the FDA more than doubled
The FDA still lacks critical information that could help mitigate shortages
The FDA lacks authority to require manufacturer recalls for most drug products
A Self-Inflicted Wound
As of April 12, 2023 the FDA database lists 201 distinct prescription drugs that are currently experiencing a supply shortage, are backordered or permanently discontinued. While some of these are infrequently used and easily substituted, others, including some antibiotics, steroids, anticoagulants, anticonvulsants, anesthetic agents can be life-saving and are not easily substituted.
While the average drug shortage lasts about 1.5 years, more than 15 critical drug products have been in shortage for over a decade. (Figure 2) Note that one third of drugs on this list are antibiotics essential in the treatment of infections.
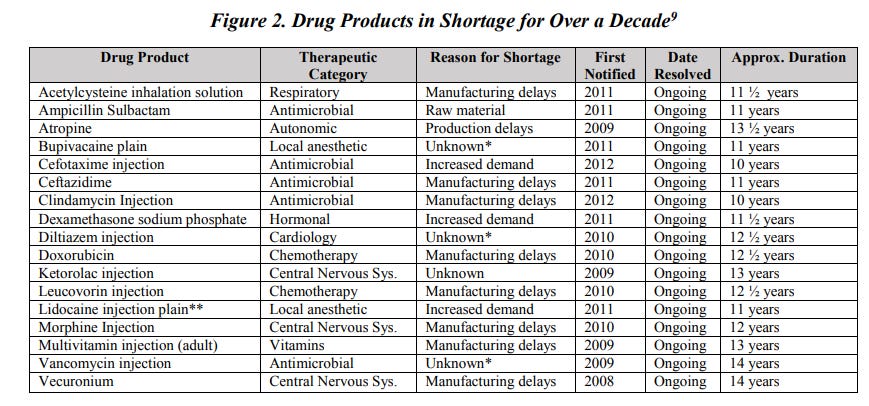
Drug shortages predominately affect older generic drug products, which account for approximately 90 percent of drugs sold throughout the U.S., but only represent 18 percent of all drug costs. Between 2004 and 2016, 40 percent of generic drug markets were supplied by just one manufacturer and the median number of manufacturers in each drug market was two.
An overwhelming majority of the world’s prescription drug supply, either completely manufactured generics or raw materials used in pharmaceutical manufacture, are imported from foreign countries. Over the last three decades, the US generic pharmaceutical market has increasingly outsourced its production to countries with lower labor and manufacturing costs in response to low profit margins. By 2021, a staggering 87 percent of generic API manufacturing sites and 63 percent of generic finished dosage manufacturing sites were located overseas. (API stands for active pharmaceutical ingredient and is the basic biologically active ingredient of any drug product).
The Administration for Strategic Preparedness and Response (ASPR) estimates that 90 to 95 percent of generic sterile injectable drugs used for critical acute care in the U.S. rely on key starting materials from China and India. The number of Chinese-based API manufacturers that registered with the FDA for the U.S. market between 2010 and 2015 more than doubled.
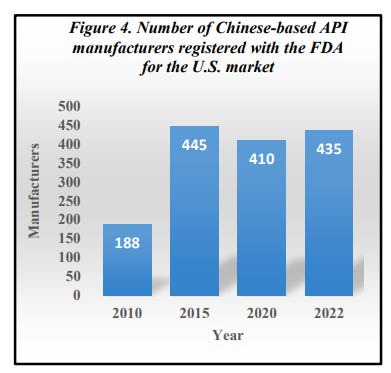
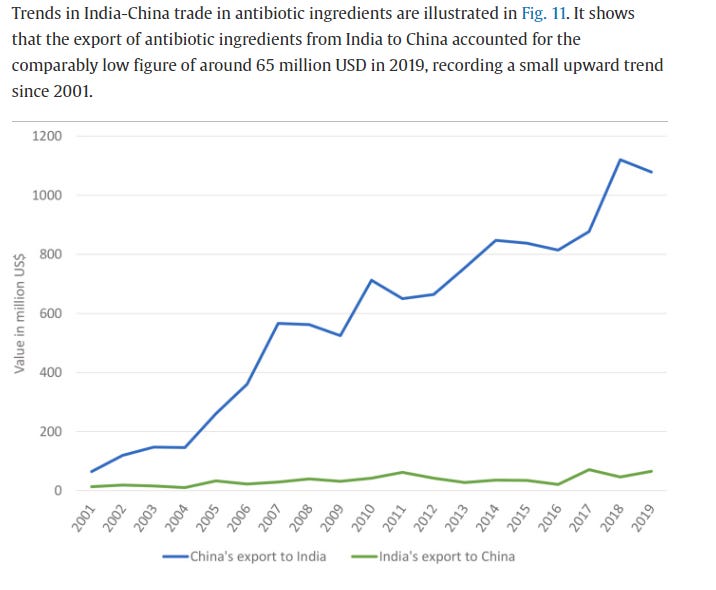
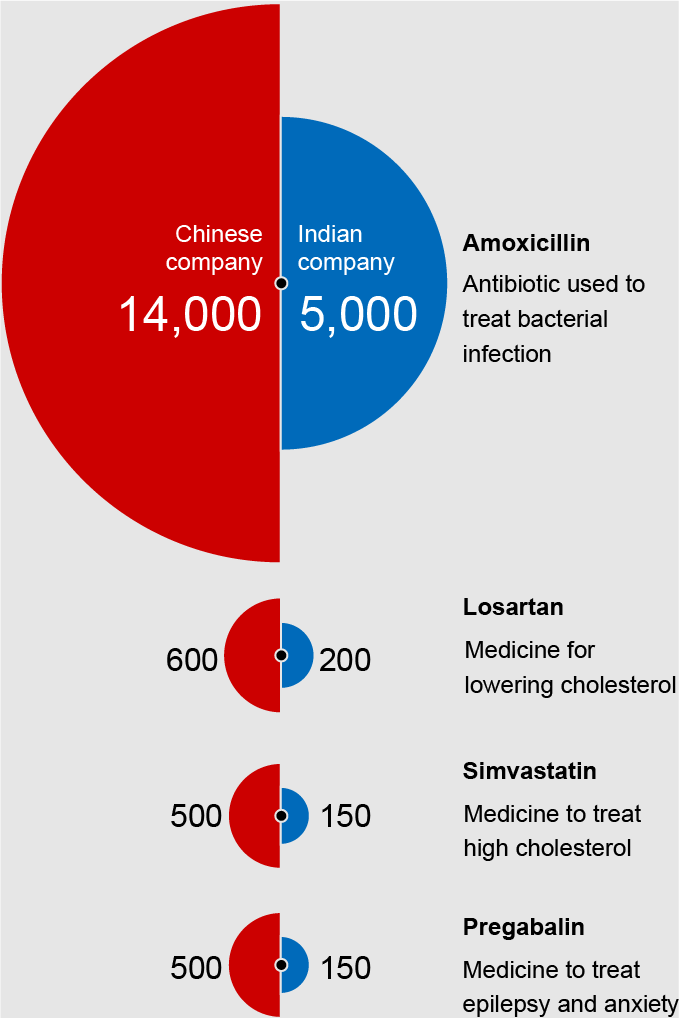
Although India is often described as “the world’s pharmacy” because it boasts the world’s third largest pharmaceutical industry, this is a misnomer because it imports nearly 70 percent of its API supply from China and without these chemicals their manufacturing capability would come to a grinding halt. Therefore, in reality it is China, not India, that is the true holder of this moniker. The graph below shows how lopsided the pharmaceutical import-export relationship between India and China is and how the disparity is steadily worsening with time. In fact, India’s increasing reliance on Chinese starting materials threatens their own pharmaceutical sovereignty.
India is dwarfed by China's production scale capacity by several orders of magnitude.
Once you trace every drug to its starting point, all roads lead to China.
Cheap Outsourced Products Kill Americans
Heparin is an injectable anticoagulant or blood thinner used for the treatment of blood clots. In 2007 and 2008, heparin sourced from Chinese manufacturers was linked to the deaths of hundreds of Americans.
In August 2022, US pharmaceutical giant Pfizer entered into a 5-year contract with Chinese manufacturer, Zhejiang Huahai, to produce and sell their blockbuster COVID drug, Paxlovid in China. The same Zhejiang Huahai was at the heart of a global scare and recall of Valsartan, a commonly used blood pressure medication which was found to contain more than 200 times the acceptable limit for the carcinogen N-nitrosodimethylamine (NDMA). Subsequently, at least two more carcinogens and a potential fourth one was linked to its Chinese manufacture. The company accepted the returned batches, then “reprocessed and released [them] to customers in non-U.S. markets” according to a scathing letter issued by the FDA, causing the agency to ban APIs coming out of their manufacturing sites. Pfizer teamed up with the company very soon after the ban was lifted.
“The companies knew it had a problem, but didn’t fix it over the course of six years,” said Rosemary Gibson, a health care expert at the Hastings Center and author of “China Rx: Exposing the Risks of America’s Dependence on China for Medicine.” “It’s not a fluke, it was a known serious problem. This company was putting a dangerous product out there for human consumption.”
The video clip below is a tour de force of the scope of the problem at hand, and although from 2018, most of it is as germane today as it was back then.
A Ticking Time Bomb, A Disaster Waiting to Happen
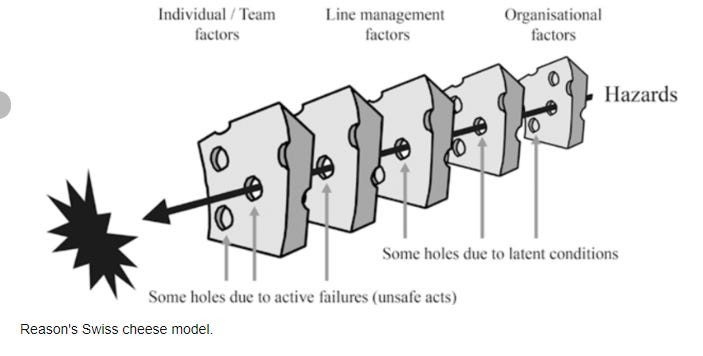
Our pharmaceutical supply chain is veritable concatenation of slices of Swiss cheese deficiencies, and it is only a matter of time before all holes line up and catastrophe strikes.
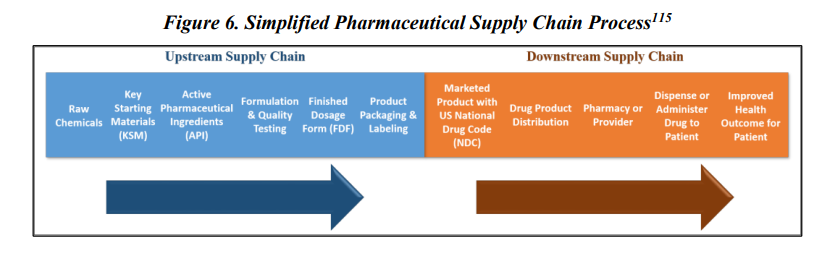
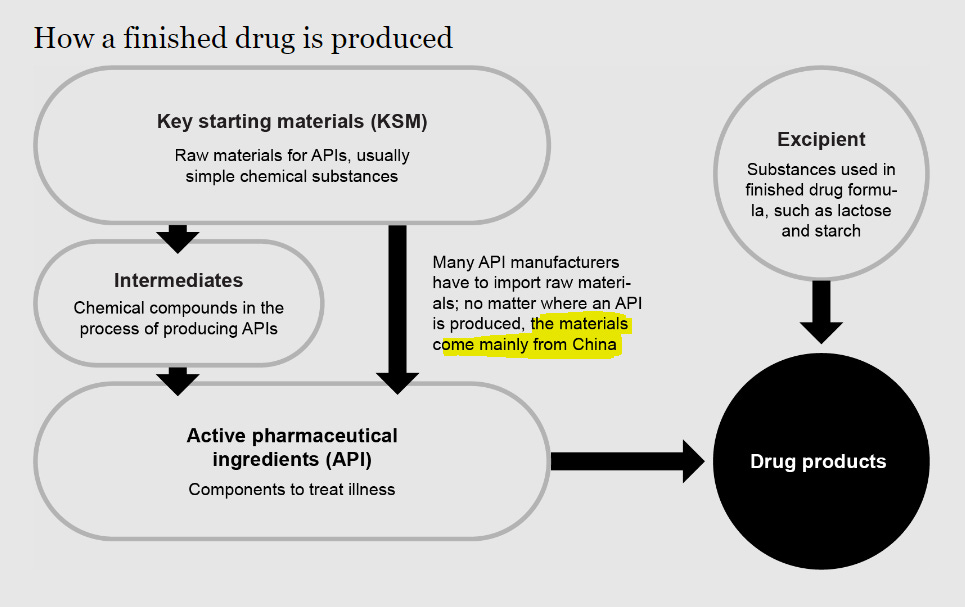
Neither the federal government nor key sectors of the pharmaceutical supply chain, including manufacturers and distributors, have end-to-end supply chain visibility, from the key starting materials (chemicals, solvents, reagents, etc.) needed to manufacture API to the intermediaries, and ultimately downstream suppliers, such as GPOs, distributors, and provider. Manufacturers do not always know where their key starting materials are from and they generally do not know the API suppliers’ full capacity as the API manufacturer may be producing APIs for multiple vendors.
The unwieldy complexity of the global supply chain and the resultant opaqueness gives a false impression of diversity in sourcing in manufacturing. In fact quite often it is impossible to know which companies are actually making the products. There could be one contract manufacturer for multiple suppliers or one API supplier for multiple finish dosage manufacturers.
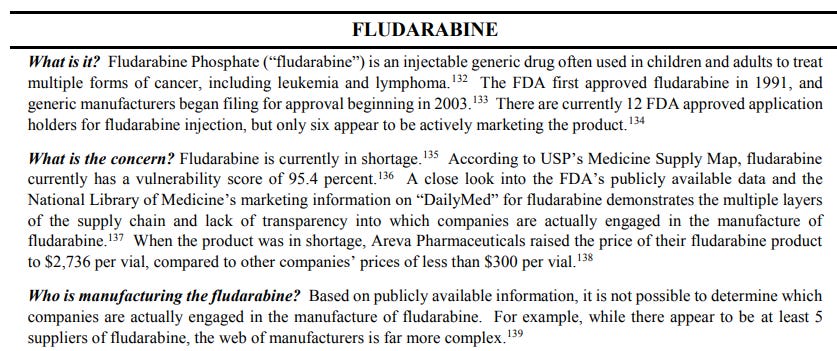
For example, tracing the origin of fludarabine, an anticancer drug often used in children is an impossible task and the winding supply maze is complex enough to make anyone's head spin.

To add insult to injury, the law doesn’t require the sources of a drug’s ingredients be disclosed. Rather, thanks to a gaping legal loophole, a drug maker can claim as the country of origin wherever the drug’s various components were “substantially transformed” into the final product. That means a pharmaceutical manufacturer can source ingredients from around the world. But if it pulls them all together into pill form in America, the country of origin can be claimed as the United States.
The COVID-19 pandemic has exposed the risks of relying on suppliers from a concentrated geographic locations of the world. As soon as a natural disaster or contagion strikes, a major chunk of the pharmaceutical supply chain gets disrupted, usually for a prolonged period of time, and ratchets up supply chain uncertainties from their already high baseline opaqueness as described above.
Starting March 2020 through February 2022, due to the COVID-19 pandemic, the FDA suspended routine in-person surveillance inspections and instead relied on manufacturers to self-report manufacturing and quality assurance records upon request.
Although the FDA did identify a number of quality problems during this 2-year interval, one can only guess how many manufacturing deficiencies were missed because of this self-reporting honor system, especially with regards to overseas manufacturers, many with severely troubled pasts and sketchy track records. Katherine Eban, an investigative journalist and author’s 2019 testimony is an eye-popping and jaw-dropping expose of depravity, deception and inveigle extant in the overseas pharmaceutical manufacture industry. She describes several instances of manipulated tests, unreported results, and loose batch records that showed the plant using expired materials. During one inspection, a stack of documents disappeared entirely; and the FDA inspector found them later on an upper floor, tucked inside a wooden crate.
The FDA does not have mandatory recall authority for all drug products. The FDA recall authority is limited to food, biological products (e.g. vaccines), and controlled substances. Therefore recalls must be voluntary with respect to most drug products.
What this means is that at the US FDA, which as a matter of routine course has little clarity on supply chain and manufacturing specifics, made matters worse by suspending in-person inspections for a full two years, and simultaneously lacks authority to mandate recall of drug products that slip through these gaping cracks in regulatory oversight. You do not have to be genius to figure out what blood curdling catastrophes could unfold if all conditions were met and the holes of the Swiss cheese lined up just right.
Is There a Cure for The Disease Afflicting the Cure?
Although there is increasing recognition of the alarming and potentially catastrophic pitfalls of a massively outsourced pharmaceutical supply chain, to date, federal and industry efforts to prevent and mitigate drug shortages have been woefully insufficient as evidenced by the ongoing short supply of over 200 prescription drugs published in the FDA’s own database.
I predicted the current scenario with alarming accuracy in my article over 3 years ago. The intervening span of time has wrought not only record pharmaceutical shortages and supply chain disruptions, but we now face a deadly fentanyl overdose epidemic of unprecedented magnitude and lethality fueled by starting materials sourced from China. I described the deadly orchestra and Symphony of destruction in another one of my articles (seem to have knack for prediction, me thinks)
What tragic irony that we are now addicts beholden to the same supply chain for the cure to our diseases while it inflicts upon us the disease of addiction.
The immense urgency of the whole situation is rendered into even sharper relief when you consider the escalating tensions between the US and China. You do not need a fancy degree that comes with 25 years of crushing student debt to know that picking fights with your drug dealer rarely ends in your favor.
Sudden disruptions to already constrained supply chains during war would lead to rationing of drugs and less efficacious substitutions and result in cataclysmic consequences by scuttling an already strained healthcare system still reeling from the after shock of our pandemic response policies.
That the United States, the foremost leader in drug discovery, innovation, and healthcare has been reduced to hand wringing helplessness at the prospect of jeopardized overseas supply chains impacting the health of its citizens is unconscionable.
A strong domestic healthcare manufacturing base is as much a matter of national security and defense as it is of public health and augurs the very survival of a nation. If China were to announce an export ban on medical exports, our healthcare infrastructure would collapse in a matter of months if not weeks.
The United States Senate committee and homeland security and governmental affairs report published in March 2023 put forth the following recommendations:
Invest in domestic advance manufacturing capabilities for critical generic drug products regularly in shortage
Conduct regular interagency medical supply chain risk assessments
Require manufacturers of life supporting in life-sustaining drug products to report increased demand and export restrictions to the FDA
FDA take steps to ensure supply chain data can be used to monitor supply chain vulnerabilities and conduct predictive modeling
Streamline private and public efforts to predict and mitigate supply chain vulnerabilities
Provide FDA with mandatory recall authority for all drug products
It can be read in its entirety here:
If there ever was a silver lining to an epidemic, it is this: it has unroofed the scabs and track marks of our self-inflicted pharmaceutical dependence and given us a preview of what a drug withdrawal feels like. If we can’t get clean and sober from our fatal addiction, we may be consigned to an interminable future of compromising our national interests in exchange for our drug fix. There’s been a lot of talk of economic renaissance by the current administration. What better way to stimulate our economy and simultaneously strengthen the country’s immunity to extraneous forces than by ensuring a domestic supply chain capable of producing items critical for the safety, survival, and health of all Americans? There never has been a greater opportunity to harness the kinetic energy of our nation’s manufacturing sovereignty and reclaim the golden age of American ingenuity and innovation. The only question is, will we?
Which one will it be, America: Carpe Diem or Crappy Diem?





We have to bring back our medicine manufacturing onshore. It’s like offshoring our war weapons to a foreign adversary…madness. I’ve lost all faith in our FDA.
FYI- Here is the valid link for the government PDF referred to in the article, as the article’s link is a 404.
https://www.hsgac.senate.gov/wp-content/uploads/2023-06-06-HSGAC-Majority-Draft-Drug-Shortages-Report.-FINAL-CORRECTED.pdf