The time when the WHO and governments the world over changed COVID policy based on science fiction
How a physician with a questionable record, a science fiction writer and an adult content model hoodwinked high profile journals and world governments and changed the course of pandemic history
The Shot
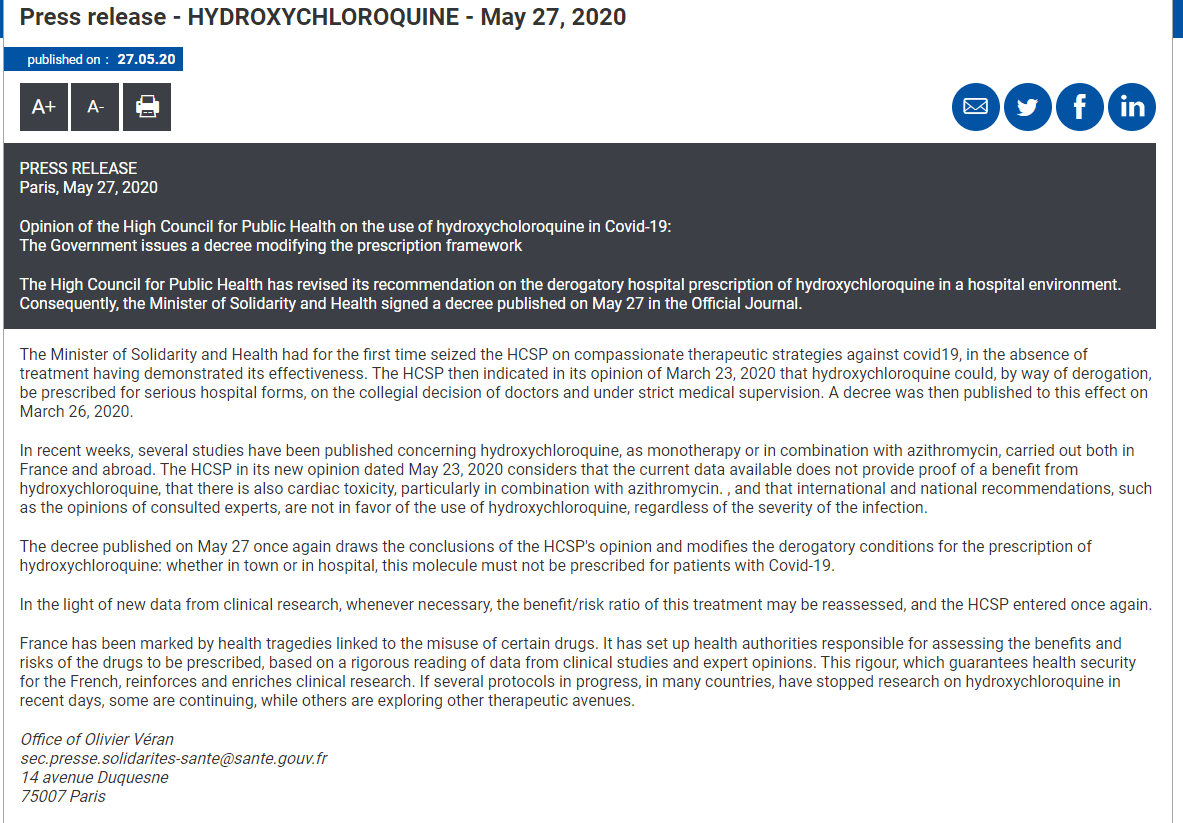
On May 22, 2020, a blockbuster paper was published in the prestigious high impact factor medical Journal, Lancet suggesting that hydroxychloroquine, an anti-inflammatory and antimalarial drug promoted by then US president Donald J. Trump and many others as therapy for COVID-19, caused increased death in patients hospitalized for COVID. Although the study was not a randomized controlled clinical trial, its claim to fame was that it analyzed the chart records of a massive 100,000 COVID-19 patients across 671 hospitals and six continents.
Just a few hours after publication, the media machine kicked into overdrive and put the blame squarely on Donald Trump’s narrow shoulders.
Within days, multiple public health agencies including the WHO and the UK Medicines and Healthcare products Regulatory Agency (MHRA) instructed organizers of clinical trials of hydroxychloroquine for COVID-19 to suspend recruitment, while the French government reversed an earlier decree allowing the drug to be prescribed to hospitalized patients with COVID.
Not wanting to be laggards to their European counterparts, the US FDA revoked emergency use authorization (EUA) for hydroxychloroquine shortly thereafter.
Almost immediately after publication, cracks began to appear in the quality and veracity of the dataset. The authors claims defied possibility and stretched credulity. Which is no mean feat when you consider that at the peak of COVID mania in the haze of confusion and chaos, the scientific community had jettisoned all pretense of critical appraisal and was voraciously consuming prodigious quantities of scientific tripe at rates that would have put hot dog eating champions to shame.
The database used for the Lancet study included a massive 96,032 patients from 671 hospitals across six continents. However, there was a slight problem. This data was accessible only to an unknown company called Surgisphere (more on that later). In addition, it had only 4 authors, which, a very astute blog post pointed out was impossible. A global multinational study of this scope and magnitude would be conducted by a collaborative group spanning multiple continents, and 50-100 authors on a study like this was the norm. The study listed the data as coming from a "surgical outcomes collaborative" which was in fact a shell corporation company, and the CEO, Sapan Desai, was listed as the second author.
This blog post was one of the first to pick up on glaring discrepancies. For example, the Lancet article claimed nearly a doubling of mortality in the hydroxychloroquine group versus control (16-24% versus 9%). This massive effect size is unheard of in modern medicine. How did hydroxychloroquine, a drug in used relatively safely for many decades for other indications becomes so good at killing so many only for this one specific indication? Also, when there is such degree of apparent harm manifesting so impressively in any clinical trial, the trial is halted prematurely on the basis of interim analyses which alerts researchers to emerging safety signals. Were no interim analyses done for such a mammoth study?
Another red flag (or more like a burning building with encircling helicopters) was the impossible level of homogeneity of the data set across 6 separate continents. If you really believe that the proportion of current and former smokers across all continents was near identical and that the same proportion of individuals take ACE inhibitors in the United States as they do in Africa and Asia, I have a bridge to sell you, and I’ll even throw in a 3-legged rainbow unicorn that farts soft serve ice cream to sweeten the deal. Real data is real messy and these numbers are fantastically unachievable even in randomized controlled clinical trials wherein effort is expended to evenly distribute such variables.
Plus, The New England Journal of Medicine had only a few weeks prior to the petard of the Lancet article published a study of nearly 1500 patients that showed no difference in outcomes—intubation or death—in individuals receiving hydroxychloroquine versus no hydroxychloroquine. How was there such a humongous difference in effect between the two studies?
Realize that none of the thought processes elucidated above require any manner of investigative journalism work whatsoever. These were simple uncomplicated calculations and logical reasoning steps that could be accomplished by anyone blessed with the ability to read and gifted with a higher than a room temperature IQ. How then did such very basic questions escape the supposedly rigorous peer review at a top tier scientific Journal?
On May 28, 2020—only six days after the Lancet paper was published—an open letter to the editor of Lancet with more than 180 signatories at research institutions around the world enumerated a laundry list of problems with the study data and analyses. In addition, readers wanted more information about the nature and history of Surgisphere, the company that claimed privileged and exclusive access to a gargantuan dataset spanning six continents and how this obscure company no scientist had ever heard of managed to obtain such a complex dataset in a relatively short period of time.
These swirling whirlpools of doubt coalesced into a maelstrom and propelled the movement to investigate the data behind the article from curious online blog posts and twitter users into the limelight of mainstream journalism. The Scientist and The Guardian jumped into the fray and flexed their enormous investigative muscles.
The Guardian uncovered even more troubling inconsistencies. The Lancet paper claimed 73 Australian deaths as of 21 April,2020. However, data from Johns Hopkins University—which tracked cases worldwide—showed only 67 deaths from Covid-19 had been recorded in Australia by 21 April and the number did not rise to 73 until 23 April. Guardian then reached out to Australian federal health authorities who confirmed that their national diseases surveillance system database was not the the source informing the clinical trial, and that the data in the paper did not reconcile with any of their own COVID hospital admission or death numbers.
The Lancet told Guardian Australia: “We have asked the authors for clarifications, we know that they are investigating urgently, and we await their reply.” The lead author of the study, Dr Mandeep Mehra, said he had contacted Surgisphere, the company that provided the data, to reconcile the discrepancies with “the utmost urgency”
This opened up the Pandora's box called Surgisphere, from whence all manner of inveigle, depravity and evil sprung forth.
The Chaser
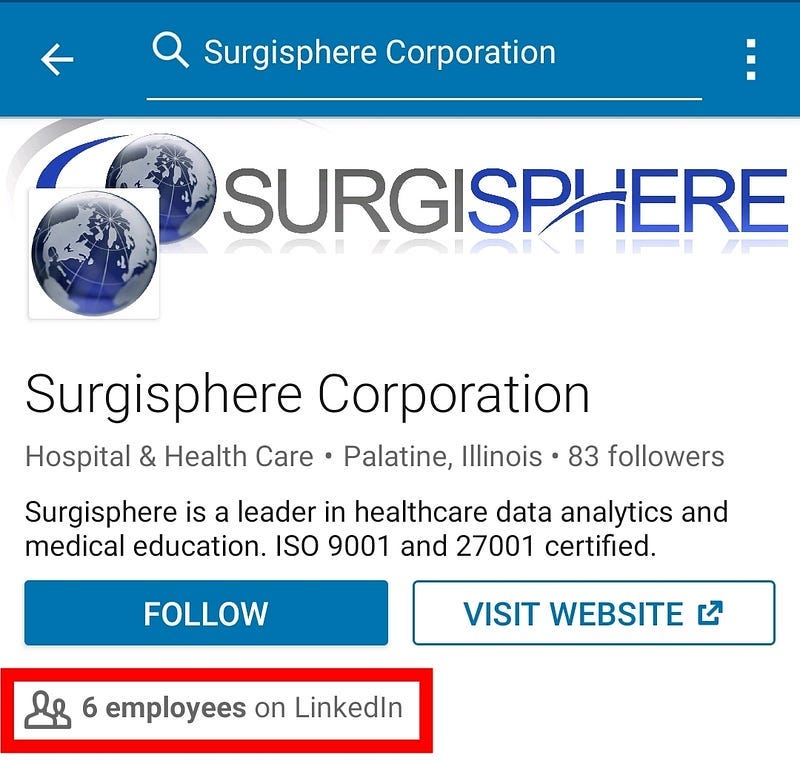
Surgisphere was a US-based company, whose handful employees (six originally according to LinkedIn, that whittled to three later) included a science fiction writer and an adult-content model in addition to its chief executive, Dr. Sapan Desai, MD. This ragtag motley crew of three was able to single-handedly provide data and analyses for multiple massive studies on Covid-19 co-authored by its chief executive, and their findings were published in two of the world’s topmost journals, the Lancet and the New England Journal of Medicine, a stupendously impressive feat when you consider that it takes Universities and Big Pharma conglomerates several hundred collaborators and several months or years to reach such pinnacles of scholarship.
Surgisphere’s employees had no data or scientific background. An employee listed as a science editor appeared to be a science fiction author and fantasy artist whose professional profile suggested writing was her fulltime job. Another employee listed as a marketing executive was an adult model and events hostess.
The “get in touch” link on Surgisphere’s homepage redirected to a WordPress template for a cryptocurrency website, which as every well heeled Ferrari driving scientist with a yacht fitted with diamond encrusted courvoisier fountains and a bevy of bikini babe shipmates knows, is how clinical trials are recruited, funded, analyzed and published.
Surgisphere’s chief executive Dr Sapan Desai had been named in three medical malpractice suits, unrelated to the Surgisphere database and had many years prior launched a crowdfunding campaign on the website Indiegogo selling a wearable “next generation human augmentation device that can help you achieve what you never thought was possible”. Needless to say, the only thing that turned out to be impossible was the ability of backers to get refunds for a non-existent product.

As the ball of yarn began to unravel, it shone light on other studies using the Surgisphere database, all of which were co-authored by Desai and the same list of authors every single time.
One study claimed that the anti-parasite drug ivermectin reduced death rates in severely ill Covid-19 patients. It was published online in the Social Science Research Network e-library, before peer-review or publication in a medical journal, and prompted the Peruvian government to add ivermectin to its national Covid-19 therapeutic guidelines.
Another study published by the same authors, this time in The New England Journal of Medicine—arguably the most prestigious medical Journal in the world— included data from Surgisphere’s proprietary (and non-existent) database of patients from 169 hospitals in 11 countries in Asia, Europe and North America and found that common heart medications known as angiotensin-converting–enzyme inhibitors and angiotensin-receptor blockers were not associated with a higher risk of harm in Covid-19 patients. Note that the list of authors for all Surgisphere publications were the same tiny group of people.
As the billowing smoke stacks from this giant dumpster fire began to spread far and wide, a nonprofit organization in Africa that had developed a tool enabling clinicians to allocate scarce ventilator resources based on a COVID-19 severity scoring system using the Surgisphere database saw months of hard work disintegrate to dust causing them to issue a hasty retraction.
(I personally test drove this tool using fictional patient profiles when it was still available online and was shocked to see it spit out similar severity scores for a 35-year-old with no comorbidities as it did for a 118-year-old with diabetes and hypertension—an impossibility that quickly disabused any notion that it used the “advanced machine learning algorithms” claimed by Surgisphere)
On Jun 4, 2020, three authors—Mandeep Mehra, the medical director of Brigham and Women’s Hospital Heart and Vascular Center, Frank Ruschitzka of University Hospital Zurich, and Amit Patel of the University of Utah—contacted The Lancet to retract their report. “They were unable to complete an independent audit of the data underpinning their analysis,” the retraction notice in The Lancet reads. “As a result, they have concluded that they ‘can no longer vouch for the veracity of the primary data sources.’” Conspicuously absent from letter of retraction was a statement from Surgisphere founder and CEO Sapan Desai.
Shortly after The Lancet’s retraction, NEJM issued its own retraction. The authors’ statement reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article. . . . We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
The Hangover
The notion that an obscure six employee US company with a website that looked like it was designed by some guy on fiverr changed the trajectory of International health policy and bring major clinical trials to a screeching halt within a span of weeks is the kind of fantastical absurdity that would’ve been rejected by Hollywood as being too unbelievable a movie plot. How then were we subjected to a real-life version of “Idiocracy: the pandemic response?”
How did two top-tier high profile journals boasting less than 5% acceptance rates and a screening process more rigorous than the Olympic games’ drug testing schedule miss a football field of flashing red lights that would’ve been clear as day to a mole with cataracts?
While a through postmortem of the decaying corpse of one of the biggest scientific retractions in modern history is outside the scope of this article (and the constraints of my intellectual prowess), a short exposition isn’t nearly as egregious.
Though the quest for speedy science has been perpetual, COVID profoundly transformed the architecture, sequence and funding of scientific research. Rather than going through the usual research–dissemination–translation sequence, testing regimes were bypassed or shortened, study sizes were reduced, and time-consuming randomized controlled trials were supplemented with observational studies, or, as in the case of omicron specific boosters, just animal data became inexplicably acceptable for approval in humans. Combine that with “warp speed” corner cutting of the regulatory processes and near unlimited funding being doled out to all and sundry created the perfect milieu for every gifted scientist, grifter and their grandmother to get in on the pandemic gold rush. Just as “warp speed” is a science fiction notion because matter cannot travel faster than light without distorting into infinite mass, science short circuiting crucial steps is likely to produce a distorted amorphous blob of gobbledegook that’s less science and more science fiction.
Although the total number of failures is mind-bogglingly protean, they can be categorized into three broad compartments:
Consider just a few of these. (I’ve eschewed complex statistical points of consideration and instead list those that need no scientific background or even knowledge of medicine)
No one in the world had access to this amazing and gargantuan database except maybe Dr Sapan Desai (if it ever existed). No one had seen it. Not the journals, not even the co-authors. Most of Desai’s coauthors admitted to having only seen summary data, and independent auditors tasked with verifying the database’s validity were never granted access. Desai claimed he couldn’t share the names of hospitals involved in Surgisphere studies due to pre-arranged privacy deals with those hospitals. It is impossible to conceive that any hospital—let alone every single one across six different continents—would agree to a contract that prevented disclosure of their participation in what was clearly a landmark clinical trial.
The first author for both retracted papers was cardiac surgeon Mandeep Mehra, an eminent Harvard University professor who works at Brigham and Women's Hospital and the only one to issue a personal statement of apology, for failing "to ensure that the data source was appropriate for this use.” Mehra said he had met another of the trio, cardiac surgeon Amit Patel, in "academic and medical circles," and that Patel had introduced him to Sapan Desai. Are we to believe that the first author had no prior knowledge of the work of his main collaborators? Was he just a patsy to provide the kind of gravitas that gets shoddy papers accepted in leading journals? (Every author on a scientific paper has to certify that they have contributed to the writing and/or conduct of the study and have thoroughly reviewed the dataset and analyses undergoing consideration for publication.)
Surgisphere claimed to have collected data from the electronic medical records of nearly 100,000 COVID-19 patients across 671 hospitals on six continents. Most countries in Asia and Africa don’t even use electronic records. How and why was such a basic fact completely ignored?
The homogeneity of the data, as discussed previously, was startling to say the least and should have raised the hackles of the peer reviewers instantaneously. How could anyone explain the miraculously homogenous dataset across continents despite known differences in demographics and underlying health conditions in those populations? (See table S3 above)
Anyone who has ever worked on a clinical trial knows that collaborating with a consortium of researchers makes herding cats look like an orchestrated military drill. How then did a motley crew of just six individuals ink contracts across six continents that required specific riders satisfying each’s unique ethical and reporting requirements, write a complex study design, get statistical resources required to crunch numbers of nearly 100,000 patients—something major universities with dedicated departments of statistics take months to do— and then write not one but two papers and get both of them published in less than 6 months after the pandemic began while in the midst of a lockdown that shut everything down? How did seasoned physicians and scientists who worked on countless clinical trials miss that the statistical odds of all of this coming together so quickly made winning the Powerball look like Taco Tuesday?
And maybe the single most important question of them all: who funded this leviathan dataset? Clinical trials and even observational studies are expensive. Where did a six person outfit get the funding for a massive transnational global study? The funding disclosure at the bottom of the Lancet article was a dead giveaway. (The Chair in Advanced Cardiovascular Medicine at Brigham was Dr. Mehra. The suggestion that he supported or funded the study was disingenuous to say the least)
Richard Horton The Lancet’s top editor since 1995, had a long standing history of wading into political waters. He cast Trump’s decision to withdraw US funding from the WHO as “a crime against humanity” and called on Anthony Fauci and Deborah Birx to resign from Trump’s coronavirus task force rather than continue to lend it their expert credibility. The Lancet even wrote, in an unusual, unsigned editorial in May, that Americans should kick Trump out of office in November. Whether the Lancet’s political stance had something to do with the hydroxychloroquine paper being published is a matter of pure speculation. And it certainly does not explain the earlier NEJM publication by the same group.
The sad part of the entire saga is not what went wrong. Because just about everything did. But that the only thing that eventually turned out right—the warp speed at which the Lancet article was published also became the speed at which it was subjected to searing scrutiny and the ensuing investigation and retraction—and was ironically fueled by the media’s unslakable fetish for politicization.
There exists a concept in healthcare called “sentinel event,” defined as “a patient safety event that results in death, permanent harm, or severe temporary harm.”
Since biomedical research directly impacts lives of patients, this was a sentinel event in the annals of scientific publishing. Unfortunately no real consequences transpired and no root cause analysis was conducted. The story has all but vanished. A google search of “Surgisphere” revealed no new articles or publications highlighting the colossal failures of this sentinel event or calling for improved processes in its aftermath. Sapan Desai, the CEO of the now-defunct Surgisphere Corporation, inactivated his medical license in one state and obtained a license in another.
The infographic below is a chronological timeline of the dizzying concatenation of failures and missed opportunities and a sobering reminder of just how seemingly easy it is to pull the wool over the eyes people formally trained in the art and science of critical appraisal. Confirmation bias is more dangerous than river blindness.
It is terrifying thought to contemplate that governments and agencies the world over, with billions of dollars, maybe trillions, in resources, countless scientists, epidemiologists, physicians and other professionals at their beck and call, and no one bothered to critically read the article and instead resorted to reflexively following each other's lead in changing pandemic policy with nary an original thought or a fart bubble of doubt. Like so much over the last three years, it was another instance of sheep bleating in unison. Thank God for the power of social media and the people that post their monomaniacal data obsessions on it.
A recent investigation involving Monash University’s health evidence unit, Cochrane Australia looked at retractions among the more than 270,000 COVID-19 papers that have been lodged online since the start of the pandemic. The 212 retracted papers investigated were cited 2697 times, a median of seven times per paper.
A quarter of these retracted papers reported clinical findings relevant to patient care – almost 90% of citations of these papers referenced the retracted paper without mentioning it had been retracted, and 80% were published after the retraction.
Another recent paper compiled 25 instances where the CDC offered demonstrably false or inaccurate information. For each instance, the researchers analyzed whether the error exaggerated or downplayed the severity of COVID-19. Of the 25 instances, 20 exaggerated the severity. In mid-2021, for instance, the CDC claimed that 4 percent of the deaths attributed to COVID-19 were kids. The actual percentage was 0.04 percent (the percentages totaled 104%) . The CDC eventually corrected the misinformation, months after being alerted to the issue.
How does an agency with an annual budget of $10 billion, nearly 13,000 employees and a stated objective of stemming the tide of pandemic disinformation make basic arithmetic mistakes, and why do they mostly go in the same direction? At least Surgisphere’s excuse was that it was a six-person fugazi basement operation peddling wares to the gullible jonesing for their next hit of the cocaine of confirmation bias.
Inaccuracies in scientific studies, methodological errors and hare-brained conclusions are not all that uncommon, and experienced practitioners take results with a grain (and sometimes a bag) of salt, rarely sacrificing common sense at the altar of new results. Science by its very nature is an error prone process of iteration, and each successive go around discards the fallacies of the last while hoping it does not introduce egregious new ones. What has changed in the last few years is the hubristic notion that science is absolute truth while the truth is that it is far from it. Whilst there is no such thing as a perfect peer review, the pandemic has exposed frailties in scientific publishing that should serve as a warning to all researchers. The Axiom "follow the science” has become euphemism for quelling dissent. Science by consensus is fiction and without the lifeblood of dissent it leads us astray with remarkable regularity.
Maybe every time we read a scientific paper it’s high time to start asking "is this evidence-based medicine or is it providence based medicine?”
If you enjoyed reading this, you will be enthralled by my ciprofloxacin article which can be found here:
Follow me on Twitter at
https://twitter.com/ShivenChabria/status/1625684754170318850?s=20
And if you haven’t signed up for my substack yet, what are you waiting for?



















The thing is that anyone who has serious followed 'climate science' over the last decade could tell you similar stories. It is just that the consequences were several steps away from directly affecting everyday life compared to Covid.