Anatomy of the Pandemic state
Part 4: EUA: The history of how Dr. Fauci obliterated informed consent, turned American citizens into lab rats and created a guaranteed market for Pharma using the pretext of emergency
Unless you were blessed with the good fortune to spend the last four years cocooned in the sweet embrace of a deep coma, you’ve most certainly heard the term “Emergency Use Authorization” (EUA) extolled ad nauseum by our public health overlords and their mouthpieces in the media.
However, what you almost certainly don’t know is that since the start of the COVID-19 pandemic, FDA has issued a staggering 600 EUAs. This represents a mind-boggling sixteen-fold increase over the total number of EUAs issued for all prior emergencies, combined. In fact, the unprecedented use of emergency authorizations is one of the key features distinguishing the COVID-19 pandemic from all past public health emergencies.
So incredulous was my reading of this 600 number quoted in publications that I personally counted the total number of emergency use authorizations on the FDA COVID EUA page. Turns out it’s more than 600. (The COVID EUA numbers in the tables below are from earlier publications and have not been revised by the authors)
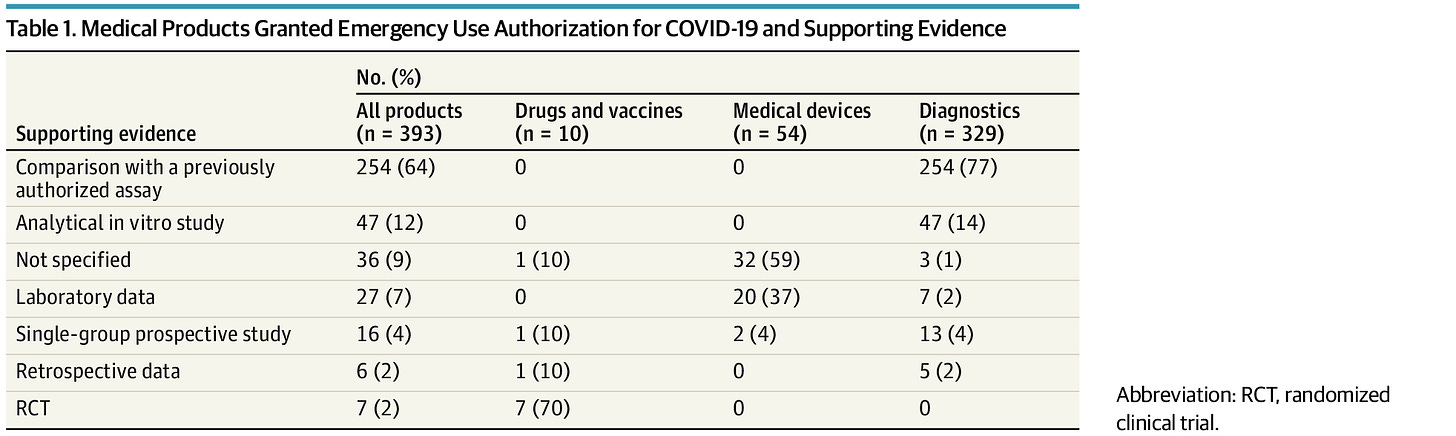
But it gets worse. From January 1, 2020 through Jan 22, 2021, 393 products were granted EUA for COVID-19 related purposes.
Over 80% of these were for diagnostic tests and 3% were for drugs or vaccines. According to one JAMA study, a shocking 59% of medical devices required no supporting evidence and most EUA's for diagnostic products were supported by comparisons with previously authorized assays rather than actual data for the performance of the products themselves. These diagnostic products were not tested against criterion standards and no evaluation showed that the products correctly identified people with versus without COVID. Most medical devices were granted EUA is without any documented supporting data and only a small fraction were supported by actual clinical data.
So what exactly is this unusual creature called EUA that seemingly materialized out of nowhere and went from being a stranger to a stalker leaving its blotchy footprints on all of public health?
In this article, we first define an EUA and then descend down the rabbit hole of how Dr. Fauci, using the template he created during the AIDS epidemic, midwifed its birth, how he incentivized hazardous bioweapons research, rebranded therapies as countermeasures and guaranteed a permanent market for pharmaceutical companies in perpetuity. You’ll see how EUA was instrumental in transforming the FDA and NIH from government agencies allegiant to American citizens into veritable wild west of Big Pharma excess in which government agencies became subservient to corporations instead of the other way around. You’ll read how EUA is the bridge that connects the original SARS COV-1 of two decades ago with the SARS COV-2 of today, and how its explosive deployment was a fulfillment of its preordained destiny: the surreptitious privatization of public health and precedence of profits before people and corporations before citizens.
What is EUA?
Under normal circumstances, FDA approval demands a high level of scrutiny and is consequently time-consuming. However, once an emergency is declared (like a pandemic or epidemic) the FDA becomes tasked with making “countermeasures” available quicker. It does so using the Emergency Use Authorization (EUA) authority under which the FDA commissioner temporarily authorizes unapproved medical products or unapproved uses of approved medical products. This mode switch that prioritizes speed over scrutiny is in a nutshell EUA.
The § 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) has three requirements for issuing an EUA:
The determination that an emergency exists by one of three specified cabinet members
Declaration of an emergency justifying the authorization of an EUA by the secretary of Health and Human Services (HHS); and
A finding by the FDA that specified statutory criteria have been met for the medical product in question. The third requirement, the FDA’s specified criteria, requires evaluating these products based on the best available evidence, weighing associated risks against potential benefits. The FDA must conclude that it is reasonable to believe the product may be effective, that the known benefits outweigh the known risks, and that there is no adequate, approved, and available alternative.
Regular readers of this publication are well aware how the 2001 anthrax bioterrorist attack was the sentinel event that catalyzed the permanent fusion of public health with biodefense. Tug hard enough at the dizzying tapestry of the pandemic state and every unravelling thread originates from this one event. It is what installed Dr. Anthony Fauci as the de facto head of national biodefense, it is what made his NIAID a beehive of public-private partnerships with dual intent biodefense-public health use and made him the highest paid bureaucrat in American history earning not only more than his own boss but even the President of the United States.
For those too shocked and scandalized with the assertions made in the preceding paragraph, you can read the origin story of the pandemic state here:
The pre-creation of EUA
The first event foreshadowing the FDA’s EUA power was the Thalidomide tragedy of the mid-20th century. In the 1950s, a new drug called Thalidomide was approved in West Germany and other countries as a sleeping aid. The drug ended up causing severe birth defects, with known cases numbering in the tens of thousands and became one of the worst public health disasters in recent times. It underscored the importance of strict standards of clinical review in approving new food and drug products, and remains a key reference point for FDA regulators, emphasizing the importance of the agency’s extensive and thorough formal approval process.
Fast forward to 1976, when reports of cases involving a new strain of influenza A (the same family of flu viruses that caused the flu pandemic of 1918) prompted fears of a possible “swine flu” pandemic. President Gerald Ford pushed for a first-ever national vaccination mandate — shortly before starting his reelection campaign. After millions had been vaccinated, vaccine associated cases of Guillain-Barré syndrome began to skyrocket creating panic and furor. Even worse, no pandemic ever materialized and this ordeal became a health policy disaster that severely undermined trust in public health expertise. If you want to read more, here’s my full-length essay on the pandemic that never was:
Next, the AIDS crisis gave rise to an early precursor to the EUA. In the late 1980s, public health experts suggested that an investigational drug called DDI might prove useful for AIDS patients. Many objected that DDI lacked formal approval and was not guaranteed to be safe and effective; others countered that the risks of breaking protocol by issuing a drug lacking formal approval paled in comparison to the number of lives that could be saved. Impatient with FDA regulators’ conservative approach, Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, proposed a new “parallel track” system to administer DDI to eligible patients while continuing to study the drug. Other measures for circumventing FDA’s formal approval process already existed at the time, but this proposal attracted the attention of President George H.W. Bush, whose support encouraged FDA to adopt the process and administer DDI to those in need.
President Clinton's Food and drug modernization act of 1997 expanded access to investigational drugs and devices during emergency situations and in effect laid the groundwork for granting FDA sweeping emergency use authorization power codified under EUA many years later. Notably, the 1997 act did not define the word "emergency," and instead left what constitutes it at the discretion of the secretary of Health and Human Services, exactly as EUA after it.
The Creation of EUA
While the pre-creation of EUA gestated under the presidency of George H. W Bush, it was birthed during the presidency of his son. The aftermath of the 2001 anthrax attacks caused a complete restructuring of our public health infrastructure. Public health became syncretized and synonymous with biodefense.
Post 9/11 anthrax, the Bush Administration began ramping up biodefense spending, which skyrocketed to $317 million in 2002. By 2003, the Bush administration was requesting $2 billion in annual budget for biodefence — a sum that exceeded the combined research budgets for breast cancer, lung cancer, stroke and tuberculosis. That same year, Bush announced in his State of the Union address that he would propose a further $6 billion for the development and stockpiling of vaccines over the subsequent decade, in addition to baseline biodefence funding.
This massive skyrocketing of biodefense spending was the driving impetus for NIAID transforming from a public health agency to the centerpiece of national biodefense. It catapulted the head of NIAID, Dr. Fauci, from the head of one of NIH’s 27 institutes to being the Director of NIH’s second-largest institute and simultaneously placed under his dominion biodefense, formerly under authority of military or intelligence agencies.
On April 4, 2003, Dr. Fauci testified in front of the House hearing of the 108th Congress favoring President George W Bush’s plan to overhaul and restructure national biodefense through the passage of the Project Bioshield act. He was one of the most vocal cheerleaders for granting the FDA sweeping approval powers through the invocation of EUA.
In the following paragraphs we will use words from Dr. Fauci’s own testimony, verbatim, to show how he seized upon this watershed moment in history to not only exalt his own powers but to gain indirect authority over the FDA from this point going forward. You’ll read how—twenty one years ago— he considered irrelevant any distinction between a natural contagion and one resulting from a lab leak or an act of bioterrorism. Important context: this hearing took place just a few weeks after the identification of the causative agent of original SARS COV-1 outbreak in China. SARS COV-1 was the pretext that greenlit the subsequent two decades, leading up to COVID-19, of what has now come to be described as “gain of function” research. Therefore, whist EUA was conceived during SARS COV-1, it was deployed only sparingly during the first two decades and its existence was largely unknown to the public at large. Indeed, the only vaccine ever approved using EUA prior to the COVID vaccines was the Anthrax Vaccine Adsorbed (AVA) and it was for sole use by the Armed Forces.
The transcript of the full testimony can be read at this link —>
Carefully read the exchange below between Rep Henry Waxman and Dr. Fauci.
Mr. Waxman: Is there a potential for dual use where the
research of biodefense may well lead us to research
breakthroughs for other diseases?
Dr. Fauci: I think it is not only a potential, Mr. Waxman,
I think it is inevitable that there will be an important
contribution to the research that we put into emerging and
reemerging diseases to inform us about biodefense research, and
it is without a doubt that the research that goes into
biodefense will help us with naturally occurring.
Because as a matter of fact, as we have discussed before,
as you know we feel that deliberately released microbes is just
another form of emerging and reemerging disease. Instead of
occurring naturally, it is done with malice and deliberately,
but the end result can be the same.
In some respects, nature itself can be our worst
bioterrorist.The casual nonchalance with with which Dr. Fauci casually dismisses Waxman’s question on dual use research created pathogens versus those occurring in nature as distinctions without a difference is shocking, especially when viewed in the rearview mirror of the last 4 years when he mocked those who dared to suggest the possibility of COVID-19 being a lab leak. Also notice how he labeled mother nature “our worst bioterrorist” just as he was becoming the most powerful man in America as the result of an instance of actual bioterrorism. Did he know something about SARS COV-1 that he wasn’t telling us?
(Intriguing Sidebar: The global outbreak of the 2002-2003 original SARS epidemic can be traced to a 64-year-old Chinese physician named Dr. Liu Jianlun who traveled in Feb 2023 to Hong Kong to attend a wedding. He stayed in room 911 on the ninth floor of the 487 room Metropole Hotel in Hong Kong. The WHO eventually estimated that roughly 4,000 of the world’s total SARS cases (about half) during that outbreak could be traced back to Liu’s stay at the Metropole Hotel. What a bizarre coincidence that of all the nearly 500 rooms in the hotel, Dr. Liu just happened to be staying in room 911 at a time when 9/11 and anthrax bioterrorism were foremost on people’s minds and used as catalysts to permanently restructure public health.)
In the excerpt below, Dr. Fauci explains how Project BioShield’s true intent was to inject massive sums of money into government funded bioterrorism research and indirectly bring the FDA—the sole agency capable of approving therapeutics rebranded as biosecurity “countermeasures” and he sits atop all of national biodefense.
Dr. Mark McClellan, the FDA commissioner and Dr. Fauci’s compatriot when EUA was first written into law, went on to sit on the board of Pharma Giant Johnson and Johnson, which just so happened to be one of the only three recipients of Emergency Use Authorization of their COVID-19 vaccine. Pure coincidence, I'm sure.
Dr. Fauci: I appreciate the opportunity to discuss Project
BioShield with you today. As you know from the legislative language, the purpose of Project BioShield is to accelerate the research, development
and purchase and availability of effective medical
countermeasures against chemical, biological, radiological and
nuclear terrorism and public health emergencies.
I am going to very briefly discuss the first two components
in the context of how they relate to the work at the NIH; and
my HHS colleague, Dr. Mark McClellan, the FDA Commissioner,
will discuss both the procurement issues and how they relate in
the context of the FDA's responsibilities....
In the following excerpt, Dr. Fauci lays out explicitly and unambiguously his vision to use the pretext of perpetual war to irretrievably restructure and fuse public health with biodefense in the aftermath of 9/11 and anthrax bioterrorism. To quote my own words “Every infectious disease came to be viewed through the lens of biodefense, and, as during every war, the liberties and freedoms of citizens were to be rendered inconsequential and even antithetical to the survival of war machine. Fauci became the equivalent of a four-star general whose mild-mannered demeanor belied the iron grip he held on every facet of the government machine and why none dared question his diktats.”
Fauci: However, the events of September 11, 2001, and the
subsequent anthrax attacks have changed, probably forever, how
the biomedical community is going to respond to emerging
threats. We are now in a wartime mode...In the passage below Dr. Fauci clearly outlines EUA’s eventual destiny. The goal was to use the cudgel of emergency to rebrand therapeutics as “countermeasures” thereby circumventing and short circuiting regulations and swapping scrutiny for expediency.
Fauci: the legislation [BioShield] provides for a number of special
authorities at NIH that will have the aggregate effect of expediting the
research process. This is what we call the push toward the
countermeasure development. Among those, BioShield provides for
expedited peer review of grants and contracts, and I emphasize
without compromising the scientific, technical and programmatic
standards. It also streamlines procurement authority, bolsters
authorities for acquisition and renovation of facilities,
expedites personal services contracts and provides flexibility
with regard to personnel authority. We feel that these expanded
authorities will considerably hasten the pathway from basic
research concept up to and including effective countermeasure
development.In the following testimony, Dr. Fauci explains how EUA was designed to be a smorgasbord of perverse incentives for pharmaceutical companies to feast on. A pharma CEO’s wildest dreams pale in comparison to what Dr. Fauci gifted them twenty years ago.
The government (and thus, you, the taxpayer) bore the cost and risk for the research and development for these so-called countermeasures while pharmaceutical conglomerates were guaranteed windfall profits through the promise of government mandated mass deployment of their products under guise of emergency conditions. Dr. Fauci took great pains to emphasize that his primary focus was ensuring the financial health of his pharma friends more so than the health of American citizens. This legislation created new market incentives for pharmaceutical and biotech companies to engage in the development of Chemical, Biological, Radiological, and Nuclear (CBRN) medical countermeasures and transformed the partnership between the federal government and industry into a shared responsibility for increasing preparedness against CBRN threats.
Fauci: These are our industrial partners that are
essential to bringing countermeasure development to fruition.
Many of those firms are willing to help in the development
of biodefense countermeasures, but the fact remains that they
are business and are not nonprofit organizations, and they need
a tangible incentive to get involved.
Now when it is evident that a given product has a potential
to make a profit, few incentives are needed to engage industry.
However, when you are dealing with a product for which there is
no guarantee of a return or for which the market is tenuous,
these companies clearly need some assurances that there will
ultimately be a return for their investment. Without such
assurances, they will simply pursue the development of other
products.
When we meet with companies, we hear one of two things.
First, they may already be involved in the early stages of
development of biodefense countermeasures on their own
initiative. They are willing to take on a fair amount of risk,
but they want some assurances if they are actually successful
that there will be a market for their product. Many state,
quite frankly, that they do not want to be vulnerable to the
vicissitudes of the cyclical appropriation process, as sound as
that is in so many arenas.
The other scenario in which we are trying to engage
reluctant companies to get involved, namely people who have
many other things to do with their efforts and with their
expertise, in this instance, we do as we are doing now. We push
with discretionary research dollars.
However, in our experience, that does not seem to be
enough. With Project BioShield, we will further be able to tell
these companies that they can partner with us such that if at
their end they meet milestones and come up with a licensable
countermeasure they have our assurances that there will be
money available to them for advanced procurement and,
ultimately, purchase.
These are examples of what we call the pull of the process.
In summary, the accelerated development of effective
countermeasures against terrorism requires a new research
paradigm and new ways to engage our industrial partners.
Project BioShield will help us meet the challenges of
bioterrorism effectively and expeditiously.Read the following excerpt in which then FDA commissioner and now Johnson and Johnson Board Member, Mark B. McClellan, explain how the success of countermeasures depended upon the government ensuring payments for products that didn’t exist.
One vital and underappreciated aspect of the pandemic state is the outsourcing of national biosecurity needs to the private sector using the pretense of expediency and accepting as manifest truth that this agility is impossible were the government to do this in house. As you can see, McClellan and Fauci were the original progenitors of this notion.
Dr. McClellan:
I do think the most critical element's there, making sure
that there's a certainty of payment, sometimes years in
advance, if an effective, highly valuable product is actually
developed, approved and delivered for use by the public in the
event of a terrorist or other emergency health threat.
Chairman Tom Davis. There is a consensus here that we just
don't have the in House capability to take this in government
and do it by ourselves. Everyone agree with that? There is no
way we could build that up in a short period of time. So we are
by necessity forced to go to the private sector to incentivize
them to do things they otherwise wouldn't do.
Dr. Fauci: Of which they do very, very well. They do it
very, very well.Thus far, you’ve read how EUA was explicitly engineered to become a permanent revenue stream for Big Pharma and to pay them years in advance for products that didn’t yet exist. Below you will read how Dr. Fauci and our lawmakers were monomaniacally obsessed with giving the pharmaceutical industry indemnity from all liability for products that had passed minimal to no regulatory scrutiny.
Chairman Tom Davis: I guess one of the differences we have
is--in the next panel, we are going to hear concerns that the
BioShield does not really afford manufacturers of the
biomedical countermeasures enough protection against product
liability lawsuits. Obviously they are going to be engaging in
research and development and manufacture of things they
wouldn't do otherwise. We are trying to get them to do it. If
they are exposed to massive lawsuits, it could bring the
company down, expose the rest of their business
Dr. McClellan: As a general matter, the administration has
expressed some concerns about problems of liability exposures
for manufacturers creating roadblocks to developing needed new
treatments, and in this case it is something that we all need
to think carefully about. We believe that there's a lot that
can be done under authority, Section 85-804 authorities, that
we have and under the Safety Act to provide protection for
manufacturers for products that are being purchased by the
government and used in these emergency situations.
Una Ryan (CEO and President of AVANT Immunotherapeutics):
Fourth, two of my colleagues have mentioned it, there must
be adequate liability protection. I am not going to go into it
further, but simply say that from the point of view of a small
company, it isn't even a meritorious legal case that is a
threat; even just the threat itself of liability is enough to
prevent investment and put small companies out of business. So
this is a risk that small companies simply can't take.
The bill introduced by Senators Lieberman and Hatch also
provides for liability protection. Their legislation offers us
protection in the context of comprehensive incentives for
biotechs, and perhaps an approach like that can be incorporated
into the BioShield concept of government-created markets that
pull firms into this worthy effort.
Mr. Waxman: Dr. Friedman, good to see you again. You have
indicated the importance of the liability protection. Why
wouldn't the government contractor defense shield you from
liability?
Dr. Friedman (CMO for biomedical preparedness, Pharmaceutical Research and Manufacturers of America): I am sorry, sir?
Mr. Waxman: You indicated that--the concern about the
potential liability companies manufacturing these
countermeasure could face, and their inability to retain
private insurance. I am trying to understand why wouldn't the
government contractor defense shield be adequate for
protection?
Dr. Friedman: This is an area that skirts my expertise in
terms of legal understanding. But as it has been explained to
me, and I believe it is accurate, the indemnification
activities that exist for many kinds of contractural procedures
are really not anywhere near as flexible or appropriate or
useful as some of the liability kinds of protections that
exist.
I believe the recent example of how smallpox has been dealt
with is a very reasonable model for us to take forward. And if
I may just expand on my answer for a moment, to answer a
question not--that you didn't address to me, but you did
address earlier, because I really feel it is worth some further
discussion.
Our feeling is that the liability protection should be
afforded not just to the manufacturer. We think there is a very
strong case for that. I am happy to further define that. But we
also believe that there should be some equitable, appropriate
consideration of the people who are receiving the product, and,
I would even add, the people who are delivering the product;
that is, the health care providers, physicians and so forth.
The reason is I think that we are operating--anytime you
have a product considered, even approved by the Food and Drug
Administration, there is a balance of what we know and what we
don't know.
At a certain point the FDA and its scientists say, we know
enough to say that this is relatively safe and relatively
effective, because there is nothing that is absolutely safe and
absolutely effective. And we have confidence when there is a
lot of information there.
Our concern is that for some of these products, because of
the difficulty of testing them, because of the fact that they
may be in the midst of development, that balance will be
shifted and we won't know quite as much as we would like to.
And there will be more unknowns about risks and benefits.
Mr. Waxman. So you think that the manufacturers should be
protected from liability to give the incentive to development
of these products, but the public that is exposed to them, that
may have some adverse effects, should also be compensated?
Dr. Friedman. Yes, sir.
While there was some discussion at the end of the testimony, at no point did either lawmakers or Dr. Fauci and his pharma colleagues discuss actual solutions for compensating citizens injured through these experimental therapies.
On May 19, 2004, the United States Congress accepted the Project BioShield Act of 2004 with a colossal majority vote. On July 21, 2004, president George W Bush signed into law Project BioShield to improve medical countermeasures protecting Americans against a chemical, biological, radiological, or nuclear (CBRN) attack. This legislation established the EUA under Section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), which permitted the emergency use of unapproved countermeasures against chemical, biological, radiological, or nuclear (CBRN) agent(s), among other authorities, when there are no adequate, approved, and available alternatives.
The FDA issued its first EUA in 2005 for use of an Adsorbed anthrax vaccine and has since issued EUAs in response to six other infectious disease outbreaks, (seven if you count monkeypox, but the linked FDA webpage conveniently omits they authorized a vaccine and several diagnostics using EUA for this disease)
As demonstrated below, the number of drugs authorized using EUA has exploded since COVID-19 and shows no signs of abating.
Why Emergency Use Authorization needs to be repealed
Every hypothetical problem related to the implementation of EUA that can possibly be conceived has already materialized:
Potential authorization of ineffective or unsafe therapeutics: EUAs allow the use of treatments without sufficient evidence of safety and efficacy. Everyone knows about chloroquine/hydroxychloroquine being revoked for COVID. However, did you know that Pfizer’s own trial found no difference in improvement of signs and symptoms of COVID between Paxlovid and Placebo? Now, why is this drug still on the market? They ran the study between 2021 and 2022, but took Pfizer 2 years to publish the results in 2024. (I’m sure it had nothing to do with the fact that Paxlovid has generated $2.7 billion in sales, right?). Then there’s the mysterious disappearance of the the J&J vaccine which was the equivalent of being taken to a farm upstate when the FDA quietly declined to renew its EUA and the CDC’s statement read: “no longer available in the U.S.”
Political interference: Political advocacy generates pressure for or against authorizing products without considering actual safety and efficacy. This is so vast one could write a book and still need a second volume. But lets take a couple of examples: mega vaccine evangelist, Dr. Paul Offit and perpetual lockdown enthusiast Dr. Zeke Emanuel published a New York Times opinion piece in June 2020 questioning the safety of COVID vaccine rollout via EUA leading up to the 2020 election. They (appropriately) pilloried the notion of an antibody response equaling immunity to infection. After Trump lost the election, Offit went back to proselytizing multiple boosters a year of the vaccines he once considered too dangerous to approve based on… wait for it… FDA approval that equates antibody responses to immunity. You can’t make this up.
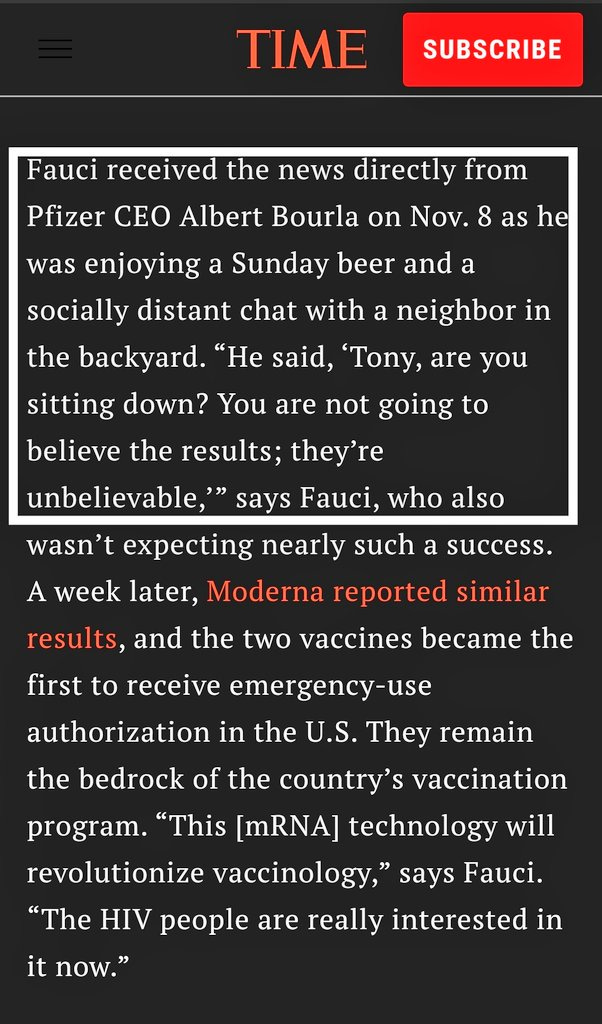
There’s evidence that the FDA and companies delayed vaccine trial results until after the 2020 US election for purely politically motivated reasons. See Vinay Prasad’s excellent analysis here. A few days after the Nov 2020 presidential election, when a Joe Biden victory was all but guaranteed, Pfizer CEO Albert Bourla called Dr Fauci on his cell phone, on a Sunday, to personally update him on the results of the mRNA vaccine clinical trial.
Now, why does the head of one of the biggest pharmaceutical companies in the world have the personal cell phone number of the head of the nation’s largest taxpayer funded research organization when taxpayers have to FOIA to access the research they fund?
(If you know anything about how clinical trials are conducted, these results were known for at least a few weeks, if not longer)
Damage to public health and agency credibility: Hasty authorizations or revocations negatively impact public health and undermine the credibility of regulatory agencies like the FDA. This one needs no further explanation. Trust in medicine, healthcare and public health is at an all time low.
EUAs are a mechanism to exploit the already incestuous relationship between regulators and those they are supposed to regulate. I have written extensively about regulatory capture and the regulator-industry revolving door that jeopardizes public safety. The full article can be read here. A staggering 10 out of the last 11 FDA commissioners—over 4 decades of agency leadership—have gone on to work for pharmaceutical companies. That includes the current FDA commissioner, Robert McKinnon Califf and three prior to him (one of which also happens to be Robert Califf). Trump’s FDA commissioner Scott Gottlieb left to join Pfizer while Fauci’s FDA counterpart during the EUA hearing , Dr. Mark McClellan, joined Johnson and Johnson and it just so happens that both Pfizer and Johnson and Johnson received EUA’s for their respective COVID vaccines. Pure coincidence, I’m sure.
Pharma receives very broad liability protection under EUA and PREP act. This liability protection, supposed to last only through the duration of the emergency, is now being extended indefinitely. As you’ve already read, liability protections for pharma were front and center during the passage of project BioShield and Dr. Fauci and his colleagues took pains to indemnify the pharmaceutical industry whilst providing no protections for injury victims who received their products. This represents a massive dereliction of duty and a colossal failure on part of the taxpayer funded public health apparatus whose only job is to protect American citizens not only from diseases but also from injurious treatments. As if that’s not bad enough, in Dec 2024, President Biden extended liability protections for Pharma for another 5 years, through 2029. This move has no basis or justification in science or public health. Note, COVID vaccines injuries are not covered under the the VICP program which pays people injured by standard childhood vaccines and shields drug makers from litigation. Instead, it is covered by another program known as countermeasures injury compensation program established to compensate injuries during public health emergencies. As of November 2024 of the 13,000 claims for COVID-19 injuries, over 10,000 were marked as waiting or in review. This sets a dangerous precedent wherein a regulatory apparatus already captured by corporate interests can be repeatedly manipulated into jettisoning all public safeguards under guise of “emergency,” obliterate ethical standards, misuse and overuse products and suffer no consequence. This too has come to fruition.
As amply documented in the preceding paragraphs, whilst the original intended purpose of EUA was to prioritize speed over scrutiny during a finite period of emergency, its length, breadth and scope were hijacked to favor special interests at significant detriment to public good. This mission creep occurred because the definition of what constitutes an emergency and the duration thereof was left to the discretion of the secretary of health and human services and public health experts, and inevitably became perverted by serious conflicts of interests and political malfeasance.
The perverse incentivization of emergency conditions has created a vicious cycle that rewards profits over people and speed over scrutiny and shows no signs of slowing down. These trade-offs are fast becoming the new normal because it significantly advantages the pharmaceutical industry bottom line, namely revenue and profits, and recuses them from all consequence. Indeed, Dr. Fauci’s testimony in favor of Project Bioshield was a litany list of perverse incentives for his pharma friends and it’s no big surprise that it accurately foreshadowed our current predicament. What tragic irony that we’ve come full circle back to the pre-regulation wild west days of the thalidomide disaster but this time wrought at the hands of a massive regulatory apparatus and their friends in high places. If past is prologue, the “avian flu” will follow the exact same template honed to perfection during COVID. Rinse, repeat.
As long as there is profit in an emergency, more emergencies will be created to profit from it. We are here now.
I know this is a long piece, so thank you for sticking around till the end. As always, your support is greatly appreciated. Be sure to read my other articles. Please share and comment and be sure to subscribe for the next installment. I look forward to reading your comments.
Recommended reading:
Katherine Watt has written by far the most comprehensive timeline of the Pandemic state that I have seen. It is mindboggling in both meticulous attention to chorological detail and the vast amount of time taken to put it together. If this is something that interests you, I highly recommend her article linked below